What is the best definition of activation energy a the energy is required to end a chemical reaction b the energy is required to bind substrate to an active site cthe energy required to form or break the bonds of reactant molecules dthe energy required to re-form bonds in products molecules. Energy free energy - physics a thermodynamic quantity equivalent to the capacity of a physical system to do work.

Solved Which Definition Best Describes The Term Activation Chegg Com
- 1841840 Joshiwa3626 Joshiwa3626 18092018 Biology Junior High School answered expert verified What is the best definition of activation energy.

. The energy required to bind a substrate to an active site C. Which is the best definition of activation energy. Activation Energy is the energy which must be provided to potential reactants.
The energy required to break the bonds of reactant molecules D. Catalysts are said to reduce the energy of activation during the transition phase of a reaction. Activation energy is the energy required by a reaction for the reaction to occur.
Moreover which is the best definition of activation energy. But it is very important to know which types of reactions require activation energy and how much. A The greater the activation energy the greater the enthalpy of reaction.
Activation energy in chemistry the minimum amount of energy that is required to activate atoms or molecules to a condition in which they can undergo chemical transformation or physical transport. Which best describes the activation energy of a chemical reaction. In this situation starting the reaction ahlukileoi and 5 more.
The energy required to form or break the bonds of reactant molecules. Activation energy is the amount of energy required to reach the transition state so A would seem like the best fit because the heat is being ADDED to reach the state. Which is the best definition of activation energy.
What is a substrate. The energy required to end a chemical reaction the energy required to bind a substrate to an active site the energy required to form or break the bonds of reactant molecules the energy required to re-form bonds in. BThe lower the activation energy the.
The energy required to end a chemical reaction B. The energy required to bind a substrate to an active site. The energy required to end a chemical reaction.
Which statement best describes how a catalyst can speed up a chemical reaction. We generally denote this energy by E. A reactant that is catalyzed by an enzyme.
Moreover in a chemical or a nuclear system so that a spark can be given to a chemical reaction or a nuclear reaction. Activation energy - the energy that an atomic system must acquire before a process such as an emission or reaction can occur. Required to re-form bonds in product molecules.
The energy required to break the bonds of reactant molecules. What is the best definition of activation energy. Which is the best definition of activation energy.
The catalyst lowers the activation energy making it easier. The energy required to re-form bonds in product molecules.
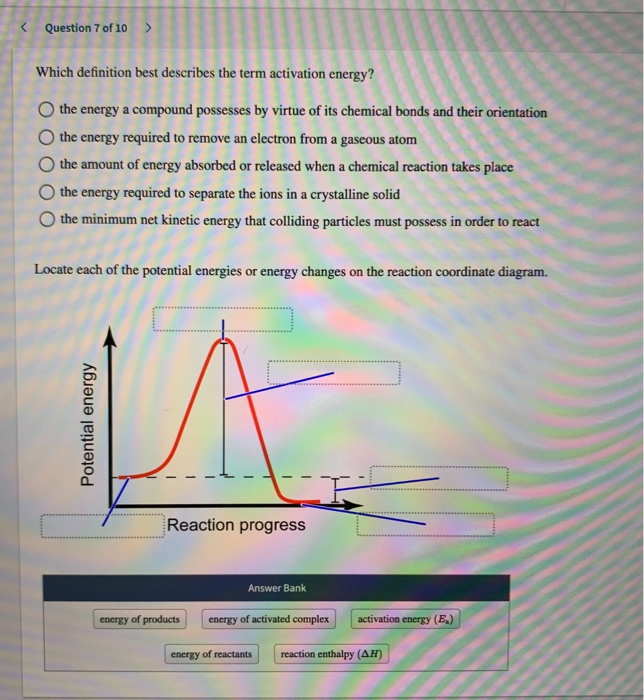
Solved Question 7 Of 10 Which Definition Best Describes Chegg Com

Solved Which Definition Best Describes The Term Activation Chegg Com

The Three Particles That Make Up An Atom Are A Protons Neutrons And Isotopes B Neutrons Isotopes And Electrons C Positives Negatives And Ppt Download
0 Comments